- Systematic Review
- Open access
- Published:
Ethical and social issues in prediction of risk of severe mental illness: a scoping review and thematic analysis
BMC Psychiatry volume 25, Article number: 501 (2025)
Abstract
Background
Over the last decade, there has been considerable development in precision psychiatry, especially in the development of novel prediction tools that can be used for early prediction of the risk of developing a severe mental disorder such as schizophrenia, depression, bipolar disorder. Although the clinical efficiency of those tools is still unclear it is crucial to consider the future ethical and social consequences of their clinical use before they are used in clinical practice. The literature on this issue is rapidly growing and represents input from scholars from different fields—psychiatrists, bioethicists etc. However, to our knowledge, nobody has produced a review addressing these issues. Therefore, the present study aims to bridge the gap.
Methods
We conducted a scoping review, allowing integration of both empirical and non-empirical studies. The research question addressed is: what are the ethical and social issues raised by the potential use of predictive tools for the risk of developing of severe mental disorder identified in the existing empirical and theoretical literature? After developing the search terms, we conducted a search in three electronic databases: Scopus, Web of Science and PubMed. For the included articles bibliometric analysis and inductive thematic coding was performed. To ensure the transparency and rigour of this scoping review we followed he Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR). A qualitative inductive thematic analysis of the included articles was performed using Atlas.ti.
Results
After screening, evaluation for eligibility and citation tracing 129 publications were included in the scoping review. The articles represent a wide range of fields of research—clinical psychology, general medicine, neuroscience, genetics, clinical genetics, psychiatry and mental health, philosophy, ethics, etc. The majority of the articles (83) are theoretical studies, 35 papers report results of empirical research and 11 are review papers. Qualitative thematic analysis of the included articles revealed four main themes: 1) Potential benefits and harms; 2) Rights and responsibilities; 3) Counselling, education and communication; 4) Ethical issues in different applications.
Conclusions
The articles included in the review cover a wide variety of concerns that might be raised when implementing predictive tools for the risk of developing of severe mental disorder. However, some important gaps in the literature are indicated. First, there are issues that should deserve more attention than they have received thus far (clinical utility, extensive or mandatory use). In several cases there is no empirical knowledge that determines whether particular concerns are justified (stigmatisation, use of machine learning algorithms).
Background
A family history is a key risk factor for many mental illnesses [1]. Severe mental illness usually refers to psychiatric or psychological problems that are so debilitating that they severely impair the ability to engage in functional and occupational activities. Examples include major depressive disorder, bipolar disorder, schizophrenia, and severe forms of anxiety disorders. Studies indicate that children born to parents with severe mental illness, such as schizophrenia, bipolar disorder or major depression, are at increased risk of developing mental health problems and one-third of this group may experience the onset of severe mental illness during early adulthood [2, 1]. For example, offsprings of parents with depression have a 40% chance of developing a depression [3].
Parents with mental illness are often concerned that their disorder may impact the wellbeing of their children, e.g. due to genetic risk or possible parenting difficulties [4]. To counteract the increased risk, preventive interventions and resilience strengthening, e.g., social support, positive parenting style or psychological counselling can be effective. However, healthcare professionals seldomly discuss their patient’s worries and the role of parenting style [5, 6].
In recent years, there has been considerable progress in developing personalised prediction models in psychiatry to predict the onset of mental disorders and the efficiency of interventions for resilience strengthening [7]. Some authors claim that “The clinical use of personalised prediction models in psychiatry is becoming increasingly feasible” [8].
However, we are not there yet and there is still a lot of work to be done. Rosen et al. in their comprehensive external validation study of 22 prediction models notice that different approaches have been undertaken recently to predict the onset of psychosis in individuals at clinical high risk. The predictors in these studies ranged from demographic and clinical data to electroencephalogram or neuroimaging features with accuracies ranging from chance (around 50%) to as high as over 90% [9]. Overall, the consensus seems that personalised prediction of future transition in the clinical or familial high risk state, is feasible, but authors warn that the development of an ethical framework is necessary [9]. Sanfelici et al. in the systematic review on diagnostic and prognostic models for patients with psychosis risk syndromes conclude that “across prognostic models, sensitivity reached 67% and specificity reached 78%” [10]. Hafeman et al. in a study on using bipolar polygenic risk score for person-level prediction conclude that that polygenic risk scores for bipolar disorder may improve person-level risk prediction, nevertheless they also emphasize that before clinical applications of these models external validation and addressing of ethical implications is needed [11].
The EU funded FAMILY project is one of the first major efforts to address these problems by establishing a multidisciplinary consortium aiming at improving the life of persons affected by severe mental illness and their families [12]. One of its aims is to develop a prediction model to inform how the joint contributions of relevant (genetic, neuroimaging, behavioural, environmental, clinical) factors from father, mother and child(ren) may increase risk for mental health problems in offspring [12]. The ultimate goal of research into risk prediction is a clinically implemented tool that uses artificial intelligence (AI) for early prediction of the onset of severe mental disorders and is based on analyses of large amounts of individual and family information—behavioural, social and biological data, acquired through questionnaires, tests, interviews and other possible sources.
The development and clinical use of this type of tools for prediction of risk of developing severe mental illness raises several ethical and social issues, such as quality of informed consent, stigmatisation, discrimination, and lack of transparency. Some of these concerns are primarily connected to the use of AI. For example, if the machine algorithms are not transparent, then physicians cannot determine the rationale behind a given prediction. This lack of transparency undermines the process of informed consent, as individuals cannot be adequately informed about the prediction. Additionally, there are valid concerns that the data used for developing prediction tools may be biased, with certain groups being over- or underrepresented, which could lead to discrimination against some groups of patients. However, not all ethical and social issues are AI related. Simply labeling a person as at risk of severe mental illness can raise concerns about discrimination and stigmatization. Many of those issues are discussed in the literature [8, 13], especially regarding the prediction of genetic risk of mental illness; however, there is no up-to-date study that maps a comprehensive overview of the concerns that have been raised so far. The aim of this review is to address this gap by providing a comprehensive overview of ethical and social issues associated with the potential use of novel prediction models for severe mental illnesses, as recorded in the current scientific literature.
Methods
To identify ethical and social issues related to the implementation of risk prediction of severe mental illness we performed a scoping literature review. Scoping reviews, according to Munn et al. are “ideal tool to determine the scope of coverage of a body of literature on a given topic and give a clear indication of the volume of literature and studies available as well as an overview (broad or detailed) of its focus” [14]. Because we intended to include both empirical and non-empirical papers in our review, conducting a systematic review had to be ruled out as there are no objective ways to evaluate normative arguments advanced in the literature. Therefore, a scoping review as described by Parsons and Johal seemed to be the most appropriate approach. As Parsons and Johal point out, scoping reviews lack the quality appraisal stage that is an important part of systematic reviews and that makes scoping review less systematic. However, due to the methodological standardisation they are still largely systematic [15].
The description of methods and reporting of this review was done following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) [16]. The review protocol was not registered or pre-published. Our review question was: what are the ethical and social issues raised by the future use of tools to predict risk of severe mental illness that are identified in the existing empirical and theoretical literature?
Three researchers (IN, NH and SM) developed the search terms which were variations on the keywords: psychosis; schizophrenia; depression; bipolar disorder; severe mental disorder/illness; major depressive disorder; psychotic disorder; psychiatric genetics; ethics; psychiatry ethics; medical ethics; research ethics; bioethics; stigma; discrimination; privacy; consent; social issues/problems; empowerment; precision psychiatry; personalised psychiatry; preventive psychiatry; predictive tools; predictive instruments; clinical prediction models; risk prediction; risk calculation; prodromal psychiatry. The literature search was conducted by IN and SM in March 2023 to identify empirical and theoretical scientific literature in three electronic databases: Scopus, Web of Science and PubMed. The full-search strategies for all three databases are presented in the Additional file 1.
The search was limited to peer-reviewed journal articles and book chapters written in English. Grey literature was not included in the review. We included all search results published before March 7, 2023, when the database search was conducted. Full books, conference abstracts and proceedings were excluded from the review. Included articles needed to address the research question by discussing ethical and social issues that might be relevant to research within the scope of FAMILY project—empirical and non-empirical articles and book chapters discussing ethical and social issues raised by the future use of predictive tools for the risk of severe mental illness.
After the database search was executed, the bibliographic data and the abstract for each article were extracted from databases, and the results were entered into the reference management tool EndNote. Duplicates were removed following the method detailed by Bramer et al [17]. As a pilot screening, two researchers (IN and SM) independently screened titles and abstracts of the first 100 identified articles to evaluate the relevance to the research question. Similarities and discrepancies were discussed to improve the screening strategy. In the rest of the screening phase, IN and SM independently screened the remaining references on title and abstract. The results were compared between the two researchers. When discrepancies were identified, a consensus was reached in a discussion. For the articles included after the title and abstract screening, access to the full text of the papers was sought via available libraries and open access publications. In cases where full text was not available via libraries and open access, the authors of publications were contacted directly to ask to share articles. If an author did not reply in two weeks, the article was excluded based on unavailability. The remaining articles and book chapters moved forward to a full-text review process.
As a next step, each full-text article and book chapter was accessed independently by two researchers (IN and SM) for inclusion or exclusion. The results were compared between both researchers. When discrepancies were identified, a consensus was reached in a dialogue.
For the included articles a qualitative inductive thematic analysis was performed by using the qualitative analysis program Atlas.ti [18]. The coding process was conducted collaboratively by two researchers (IN and SM). The process began with an initial reading and familiarisation phase, where both researchers independently read the first 10 articles to gain a preliminary understanding of the themes and concepts within the texts. Then, specific codes were identified which were grouped into broader themes. The researchers refined the coding tree through discussion to ensure it was comprehensive and clear. After establishing the initial coding tree, the researchers proceeded to code the remaining articles independently. Each researcher used the agreed-upon coding tree for coding. During this independent coding phase, the researchers occasionally identified new themes or codes not initially covered by the coding tree. To address these new codes, they reconvened to discuss and amend the coding tree, ensuring it remained relevant and comprehensive. Regular meetings were held to discuss progress, resolve discrepancies, and update the coding tree as needed, maintaining a consensus-building approach to ensure both researchers agreed on any changes to the framework. Coding was performed, and codes were compared in an iterative process. Following the coding, researchers identified and named broader themes by grouping related codes and examining patterns and relationships among the codes.
In addition, to get a better grasp of the included articles we conducted a bibliometric analysis by using bibliometrix package in R and VosViewer [19, 20]. Unfortunately, two papers [21, 22] from the collection are not included in the Scopus and therefore we were not able to include them in this analysis.
Results
After the full-text screening and evaluation for eligibility, 120 publications were included in the scoping review. Additionally, citation tracing was performed by reviewing reference lists of the retrieved full-text articles and looking up the papers that cite the retrieved articles. This resulted in 9 additional articles included in the scoping review. For the PRISMA flowchart illustrating the process of eligibility screening, see Additional file 2.
The review included 129 articles and book chapters (see Additional file 3 for the full list of the included articles and book chapters). The articles represent a wide range of fields of research—clinical psychology, general medicine, neuroscience, genetics, clinical genetics, psychiatry and mental health, philosophy, ethics, etc. The majority of the articles (83) are theoretical studies, 35 papers report results of empirical research and 11 are review papers. The distinction between review papers and theoretical papers, however, is blurred. The earliest paper was published in 1989, the latest—in 2023. To determine the most influential papers in the set several measures were used. The list of most global cited documents (Table 1) shows the papers that have received the most citations in total.
This, however, does not mean that these are the most relevant studies on the topic of the review. For example the by far the most cited paper is by Graham et al. [23]. It is an overview of the current applications of AI in healthcare and the recent research of AI specific to mental health. Therefore, the high number of citations might (at least partly) be explained by the wide area of topics it addresses and by the recent surging interest in psychiatric AI applications. The same applies to the article by Chekroud et al. [24]. It is also worth to notice that all papers (except Meiser et al.) in the Table 1 are published in journals on psychiatry (Current Psychiatry Report, World Psychiatry, Schizophrenia Research, JAMA Psychiatry, Journal of Affective Disorders) and genetic research (European Journal of Human Genetics) and not, for example, in the field of bioethics or sociology of medicine. The citation count differs across disciplines and papers in life sciences are in general more cited than papers in social sciences or humanities [25]. This at least partly explains the absence of bioethics papers in Table 1. Alternatively, we investigated the list of most locally cited documents, i.e., the papers that are most often cited by other papers that we selected for the review (Table 2).
Table 2 lists ten most cited papers within the collection of articles that we included in the review. As we see, some papers have disappeared (e. g. Graham S, 2019; Fusar-Poli P, 2021; Chekroud AM, 2021), some improved their position (e.g. Corcoran C, 2005; Meiser B, 2005) and some new papers made the list (e.g. Laegsgaard MM, 2008; Hoge SK, 2012). Interestingly a couple of papers that are published in bioethics journals appear in the list as well (Lawrence RE, 2016; Mittal VA, 2015). This list of most locally cited papers has a better chance to represent the list of most influential papers on the topic of the review as it includes the papers that are more focused on the topic of our interest. However, provided the various limitations (e.g. the differences in citation practices between disciplines, the bibliometric analysis is based only on that data from SCOPUS) this claim should be taken with some reservations.
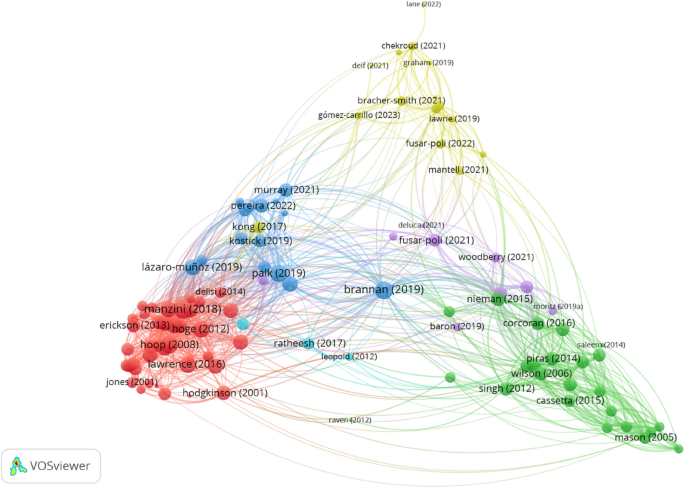
To get additional insight into the relations between the articles and themes that are covered in them, we utilised VOSViewer to produce a map of bibliographic coupling relations (Fig. 1). Bibliographic coupling tracks relations between the documents. Two papers are bibliographically coupled if there is a third paper that is cited by both papers—“bibliographic coupling is about the overlap in the reference lists of publications. The larger the number of references two publications have in common, the stronger the bibliographic coupling relation between the publications” [20]. The idea behind bibliographic coupling is that two publications sharing common references are also similar in their content [26]. The bibliographic network in Fig. 1 consists of nodes (dots) and edges (connections between dots). The diameter of the nodes and the thickness of the edges corresponds to the number of the links that the item has with other items. Further, the location of a node is determined by relations of a paper to other documents. There are 107 nodes in the map in Fig. 1 (to reduce the noise, the minimum number of citations of a document was set to 5) and setting the resolution parameter to 1, VOSviewer algorithm assigned the papers into a network of six clusters that are represented by different colours.
The largest cluster (Cluster 1), in red,—consists of 40 papers and (bottom left corner in Fig. 1). The green nodes in the bottom right corner are papers assigned to a considerably smaller Cluster 2 (24 papers). Further, there are two clusters of equal size, namely 15 papers each—Cluster 3 (blue nodes right above Cluster 1) and Cluster 4 (yellow nodes in the top centre part of Fig. 1). The purple dots in the centre of the map represent Cluster 5 that consists of 10 papers. Finally, there is a tiny Cluster 6 that consists of only 3 papers.
In some cases the decisions made by the VOSviewer algorithm are difficult to interpret. For example, Cluster 1 consists of closely related papers that address several ethical issues of genetic testing in psychiatry. However, the papers in Cluster 2 seem to address the same topic, but they are put into a different cluster. On average, the papers in both clusters are published around the same time—the mean year of publishing is 2010.7 for Cluster 1 and 2010.2 for Cluster 2. At least a partial reason behind putting the papers into two different clusters might be the higher proportion of empirical papers in Cluster 1—more than a half (18) of all (30) empirical studies are assigned to Cluster 1. By comparison only three empirical studies are assigned to Cluster 2. Further, it is also not easy to see how the topics addressed in Cluster 3 differ from Cluster 1 and Cluster 2. The nodes of Cluster 3 are wider spread than the nodes of Cluster 1 and Cluster 2 and in the map it is located closer to Cluster 1. However, on average papers in Cluster 3 are published more recently (2020) than the papers in Cluster 1 and Cluster 2. Moreover, if one takes a look into the list of authors of the papers in Cluster 3 then it becomes obvious that for a considerable proportion of articles are authored by an overlapping team of researchers (see e.g. [27,28,29,30,31,32]). Therefore, although the papers in this cluster are more oriented towards issues in research ethics, their being in the cluster at least partly might be explained by the citation practices of the authors. By comparison, Cluster 4 and Cluster 5 present less problems of interpretation. The papers in Cluster 4 address the issues of using machine learning (ML) algorithms in psychiatry and they talk about them using the framework of precision psychiatry. This is also the most cutting edge cluster on the map—the average publishing year of the papers is 2021. Finally, the papers in Cluster 5 address risks of genetic information and especially stigmatisation.
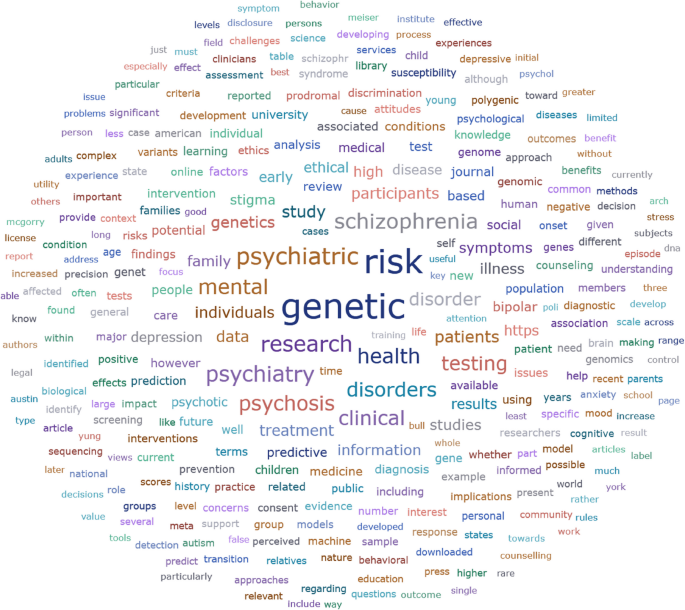
A significant part of the articles included in the review focus on genetic testing/screening for mental illness risk. The word “genetic” is the most used word according to Atlas.ti word frequencies analysis of the documents included in the review (see Fig. 2).
This might be explained by the attention that genetic testing attracted during the period the majority of the articles were written. Moreover, several papers use the term “genetic testing/screening” by not distinguishing specifically between diagnostic and predictive testing.
Qualitative thematic analysis of the included articles revealed four main themes: The themes and codes are presented in Table 3 and described in detail in the sections below (Table 3).
Potential benefits and harms
Benefits
Early prediction of severe mental disorders can bring several benefits. Some of them can be characterised as medical. For example, virtually all articles that discuss the benefits of predictive information point out that early risk prediction would enable the use of preventive measures and therefore reduce the risk or postpone the onset of a disease [33, 34, 21]. This is highly relevant to children and adolescents [35]. In case of psychosis risk prediction, the individuals may benefit from regular targeted follow-up which can help to detect the early signs of onset of psychosis. This also might help to minimise the duration of untreated psychosis which in turn improves clinical outcomes later [36, 37]. Besides those effects, many authors emphasise benefits that are not (at least directly) medical. Some authors mention that predictive information positively affects the behaviour of persons. For example, the information on risks makes persons more willing to seek professional help and change their health-related behaviour [38, 33].
A rather long list of potential benefits from a series of qualitative and quantitative studies on genetic testing of familiar risk of a psychiatric disorder is compiled by Meiser et al. [39]. All studies included in their review (except one which involves research participants who have undergone genetic testing for risk) are based on hypothetical future genetic testing scenarios. The review indicates that there is a belief that genetic testing in adults may help individuals to accept their condition and make them more willing to seek treatment, monitor their behaviour and change it accordingly or avoid triggers. They also note that one of the most widely documented benefits of genetic testing is the “potential to engage in preventive measures” [39]. Another important finding in the review by Meiser et al. is a belief that genetic testing might validate the status of mental illness alongside physical illnesses and this in turn could help to decrease social stigma that is associated with mental disorders. The rest of the benefits in the review might be subsumed under the category of control, namely, genetic testing improves the basis for planning for the future, i.e., the information might help people to make financial decisions, decisions about marriage and procreation, etc.
A qualitative study by Lawrence et al. based on semi-structured interviews with young adults at high risk for psychosis about hypothetical genetic testing provides some evidence that a test that indicates an increased genetic risk can cause positive emotions such as happiness and an increase in self-esteem [40]. Manzini and Vears also point out that some authors have proposed that predictive psychiatric genetic testing in minors allows a family to be better prepared to recognize and prevent the illness, detect early symptoms, reduce anxiety and have positive effects on family relationships. For example, it creates an opportunity for the family to discuss the condition openly. Moreover, there is evidence that some parents believe that predictive genetic testing allows them to prepare better for caring for their at-risk child [35]. Finally, several empirical studies indicate that there is a demand for predictive psychiatric genetic testing. For example, Laegsgaard and Mors in their study of psychiatric patients and their relatives report that 83% of the patients and 75% of relatives would choose to have a genetic psychiatric test, some of them (38% and 30% respectively) even if there are no options for a treatment. [41] They point out that other studies have reported similar results—“All in all, 75–99% of respondents in these studies express positive attitudes toward psychiatric genetic testing, indicating a highly positive attitude to psychiatric genetics among persons suffering from bipolar disorder or schizophrenia [..] and patients suffering from a broad range of mental diseases"[41]. Meiser et al. report a similar result. In the studies of adults they have reviewed, between 63 and 85% of participants expressed either willingness to test or a positive attitude towards testing [39].
Risk of misinterpretation and bias
When discussing the development of polygenic risk scores for prediction of mental illness, several authors emphasise the importance of using diverse and inclusive samples to avoid creating inequalities in the predictive accuracy of the models [13, 8, 42, 43, 29, 44, 23]. As Palk et al. observe, “the majority of GWAS have been conducted in high income countries, and, even within these contexts, have included mostly participants of European ancestry"[42]. As a result, the predictive accuracy of the polygenetic risk score is much higher in these populations. This problem is exacerbated further when machine learning algorithms used in the prediction models are trained only on the same populations. This issue is discussed below.
Further, another concern is the risk of false positives and negatives. False positives are prone to change the risk–benefit ratio of risk prediction by subjecting individuals to unnecessary risks without bringing any benefits. Corcoran et al. note that in the case of screening individuals at risk, false positives create a risk of over-treatment [45, 22]. Individuals are unnecessarily exposed to the stigma of provisional diagnosis [21] and other harms, such as anxiety and “needless avoidance of developmentally appropriate challenges” [46]. Lawrie et al. add to the list also potential negative consequences for employment and obtaining insurance, adverse impact on relationships, self-imposed restrictions, prejudice and discrimination [47]. That said, there are also risks of false negatives such as delayed treatment, exacerbating the condition through illicit drug use and stress [47] and a false sense of confidence [48].
Some papers raise concerns about mistakes made in the interpretation of the results. So, for example, Chapman writes that regarding the use of polygenic risk scores, the potential of mis- or overinterpretation by healthcare providers, patients and consumers is a significant challenge [43]. The consequences of that might be overdiagnosis and overtreatment. Several conditions can contribute to misinterpretation, e.g., individuals may lack necessary probability literacy and risk numeracy [43, 49], and they overestimate the role of genes in causing the condition. In other words, as a consequence of learning more about genetic predisposition people may disproportionately discount the role played by environmental and lifestyle factors [43]. Ahlgrim et al. suggest that “high-risk populations are inherently vulnerable to having their judgment clouded by the promises of preclinical detection” [50]. This might create unjustified optimism and unrealistic expectations about the benefits of testing and early interventions [49]. It is worth to note that these consequences are mostly theoretical speculations of the authors. This does not mean that they are unfounded. But there is a good reason to justify them with empirical data.
Risks of AI use
The risks of AI use in the prediction of severe mental disorders are not addressed as extensively as other topics. The reason for this is obvious—the ethics of AI use in medicine is still in its infancy. Most papers are concerned with implications of using machine learning algorithms and the resulting bias. Fusar-Poli et al. observe that ethical concerns are raised by “high complexity” and “poor explainability” of so called “black box” multimodal clinical prediction models, which “do not allow for backtracking of the key patterns that produced a specific prediction” [13]. Martinez-Martin et al. raise concerns that physicians who do not know the rationale behind the generated predictions will not be able to assess and justify their treatment decisions [51]. This in turn may jeopardises patients’ ability to give informed consent. Further, an increasing reliance on machine learning algorithms may turn them into something that is more than just support tools and encourages “defensive medicine at the expense of patient autonomy” [8].
Another concern expressed by several authors is that the machine learning algorithms reinforce already existing racial, socioeconomic and other biases [51, 52, 36, 53].
In the literature, this is called “algorithmic bias” [44]. Already existing health disparities contribute to algorithmic bias and as Walsh et al. observe, if not prevented, this may create a “harmful feedback loop” when “existing disparities may lead to unrepresentative training data. This bias may seep into predictive models, which further exacerbate disparities owing to biased predictions for certain minorities and vulnerable segments of patient populations” [44]. Therefore, for example, Lane and Broome claim that researchers must ensure that sample populations at every stage of research should be diverse and inclusive [8]. Dwyer and Koutsuleris in their scoping review on translational machine learning for child and adolescent psychiatry note that such algorithmic bias has been demonstrated with real-world consequences for ethnicity and gender. Such mistakes must be “investigated and then mitigated or transparently described before model deployment” [54]. This, however, is difficult due to the increasing speed by which the data is made available to researchers. As Fusar-Polli et al. observe: “The speed by which technology is making Big Data available to biomedical researchers is outpacing the development of new analytical techniques to understand the implicit process that leads to their generation” [13]. Therefore, they suggest that in the process of development, validation and implementation of precision psychiatry models “bias-aware interdisciplinary research and innovative practices” will be necessary to avoid different biases. However, Martinez-Martin et al. note, that “with proper calibration, the algorithm can be used to reduce bias in health care” [51].
Finally, Starke et al. claim that the use of algorithms could cause harm directly, namely, by producing erroneous predictions. As an example, they mention the IBM’s machine learning-based computer system Watson which produced unsafe recommendations for treatment. Such errors, they say, are especially worrying if the algorithms'recommendations are readily accepted by physicians [53]. This worry might be especially relevant provided the feature of the “black box” algorithms mentioned above—namely, the predictions of those algorithms are not transparent and cannot be assessed by the medical staff. Consequently, for example, Fusar-Poli et al. suggest that to increase transparency and ethical acceptability of predictions, transparent “glass-box” alternatives that are explainable and interpretable should be preferred [13].
Inequalities in access to health care
One of the concerns discussed is the cost-effectiveness of research and the implementation of predictive models into health care. The main concerns are whether the research and use of predictive models will be effective and sustainable [55, 56] and how their implementation might affect the overall distribution of healthcare resources in the already underfunded mental care [57, 13]. At the moment there is no “solid demonstration of precision psychiatry over standard approaches” [13], but there are good reasons to expect that the costs are high, and subsequently will take away the resources from other modalities. This is a serious issue especially if one considers that in many countries mental health research and treatments are underfunded [58]. Further, some authors have expressed concerns about the accessibility of the service to marginalised populations [13, 36]. Moreover, as Fusar-Polli et al. observe: “Growing digital divides can also amplify disparities in the accessibility of clinical prediction models in economically disadvantaged and marginalised populations"[13].
Risk of discrimination and stigmatisation
Ethical issues around stigmatisation are most often discussed in the papers in our review (see Fig. 2). One of the most prominent concerns is the worry that testing for severe mental illnesses increases stigmatisation of the people at risk. Ruhrmann et al. note that “The risk of early stigmatisation, negative labelling, and stereotypes has been voiced repeatedly by the opponents of preventive efforts, especially with regard to early detection” [59]. The concerns about increased stigmatisation in the context of early prediction are pressing as individuals with psychiatric disorders are highly stigmatised [60], more so than those who suffer from somatic disorders [61, 60] Some empirical studies indicate that genetic explanation of mental disorders tends to increase stigma, in particular—it tends to increase social isolation and reduce empathy and understanding amongst healthcare professionals [60].
Further, individuals with psychiatric disorders often suffer from self-stigmatisation, i.e., they internalise the stigma which makes them perceive themselves as less valuable. Brannan et al. refer to empirical evidence that the stress associated with mental stigma, including self-stigma can increase the rate of transition to schizophrenia. Moreover, self-stigma can demoralise individuals and demotivate them to pursue employment and other life choices [60]. To avoid stigma, individuals try to conceal their mental health status which in turn might significantly impede their access to mental health care [60, 62]. Besides, one must take into account associative stigma as well, which affects the family members of the individuals at risk. Marriot and Broome in their paper refer to a study, according to which “the prevalence of associative stigma might be as high as 86%, with family members reporting feelings of depression, guilt and shame” [22]. Finally, as some authors point out, there is a risk that healthy individuals will be unduly labelled as being at risk, and they will be stigmatised and, probably, offered unnecessary treatments. This, as Marriot and Broome point out “could contravene the principle of non-maleficence” [22].
However, the picture seems more nuanced than that. Some authors claim that genetic testing in fact might decrease stigmatisation by promoting the idea that mental disorders have a biological component and therefore are similar to somatic disorders. For example, in a study involving individuals suffering from depression, some participants expressed the view that a genetic explanation decreases stigma associated with the condition and changes the way other people perceive depression [63]. There is evidence that many psychiatric genetic researchers hold a similar view. In a study conducted by Erickson and Cho, the majority of researchers in the field of psychiatric genetics that they interviewed, expressed a belief that “greater biological understanding of BP [bipolar disorder], MDD [major depressive disorder], and schizophrenia will reduce the stigma” [64]. In addition, understanding that those disorders are biological conditions like other somatic diseases “would help to reframe mental illness as a condition outside the individual’s control, thus reducing guilt or sense of responsibility” [64]. Meiser et al. in their review found that the overwhelming majority of the studies looking at participant’s perceptions of the impact of genetic explanation on mental illness-related stigma show that individuals believe that a genetic explanation would alleviate stigma [39]. Bartolotti and Widdows on the basis of similar evidence claim that “Testing can promote knowledge about a psychiatric condition and decrease stigma among those who obtain such knowledge” but admit that regarding the wider social context the stigma would remain a danger [65]. However, Brannan et al. mention a systematic review which shows that public understanding of the biological component of psychological disorders has substantially increased since 1990, but this has not helped to reduce the stereotype of individuals with schizophrenia as socially dangerous. Moreover, the social acceptance of such persons has even decreased [60]. Therefore, it still might be the case that the promotion of a biological explanation of mental disorders runs the risk of exacerbating stigma.
It is important, however, to bear in mind that—as Brannan et al. point out [60]—the majority of studies on mental disorder-related stigmatisation examine attitudes towards individuals with a mental disorder. It is likely that attitudes towards subthreshold or asymptomatic individuals would be similar, however, currently, there is not enough evidence for that.
A further concern is that stigmatisation is associated with risks of discrimination. For example, stigma explains part of the higher levels of unemployment than the general population in people with schizophrenia [60]. Also there is the potential threat of discrimination by insurance companies [66,67,68, 13, 60]. A study conducted by Nwulia et al. indicates that individuals of African descent are more concerned about the risks that genetic information can be used in discriminatory ways [69]. Finally, Marriott and Broome raise concerns about the risk of epistemic injustice [22]. The term is coined by Fricker and it refers to “wrong done to someone specifically in their capacity as a knower” [70]. Due to negative stereotypes and stigmatisation people with mental disorders might be unjustly treated as less credible both when they give knowledge or interpret their experiences. The issue of epistemic injustice in psychiatry has been discussed by Crichton et al. [71], however, Marriott and Broome raise this concern, regarding the individuals at risk [22].
Risk of commercialisation
Somewhat surprisingly, there is relatively little discussion on the risk of commercialisation of predictive tests for severe mental disorders in the literature. Eleven papers mention the topic. The main concern expressed by is the premature commercialization of predictive tests for psychiatric disorders, benefitting the product owner while the tests lack the necessary precision [64, 43, 72, 73]. Chapman raises another pertinent issue: the potential involvement of commercial entities in development or production of novel prediction tools that might lead to conflicts of interest [43]. This concern encompasses the necessity of implementing appropriate oversight mechanisms to avoid influence of commercial interests—bias in training of end users, downplaying limitations of prediction tools or alternative options, influence on clinical decision-making etc.
But commercialisation is not the only worry. The psychiatric genetic researchers interviewed by Erickson and Cho expressed their concerns regarding direct-to-consumer testing because the currently available genetic predictive tests for bipolar disorder, schizophrenia and major depressive disorder are “weak and nearly meaningless”. Thus, if offered directly to the customers, several harms might be caused. At best, it would be a waste of their money, at worst—it would be a cause of worry, feeling doomed and stigmatised, which is especially harmful for a psychiatric patient [64]. Further, some researchers expressed worries that direct-to-customer testing has a negative effect on the whole field of psychiatric genetic research as society would become sceptical of the entire field [64]. Another worry voiced by researchers is that such testing is dangerous because “people do not have a broader understanding of the significance of the results” [64]. As a consequence, people misunderstand the results and make wrong decisions on the information available to them [64, 73]. However, few researchers were more optimistic about direct-to-customer testing. They pointed out that it would prevent scientists from “taking overly paternalistic approach toward the general public” and that it would allow people to make their own decisions about what kind of information they prefer to have [64].
Response to predictive information
Numerous authors have voiced concerns regarding the potential harm that may arise as a consequence to individual’s reactions to the predictive information. For example, Bunnik et al. discussing differences between genetic testing for psychiatric and somatic diseases point out that knowledge of genetic risks for psychiatric diseases could potentially undermine a person’s sense of integrity and well-being before the appearance of symptoms. Further, they mention that a positive test result itself might serve as a trigger event for the disease tested for and “the manner in which the disease is understood by the patient (e.g. “it is in my genes, therefore it is an inevitable aspect of myself”) is likely to reflect on and modulate the development of the disease itself” [61]. Similar concerns are mentioned by other authors as well. Cassetta and Godhari point out that screening for psychosis vulnerability might have potentially negative effects on personal identity: “For example, persons who are identified as being vulnerable for psychosis may begin to adopt a “patient” identity, which may lead to further exacerbation of their current psychotic symptoms. The new label may impact their future goals and aspirations, the level of support received from family members and friends toward achieving their goals and aspirations, and their levels of anxiety, which could affect the ability to meet those future goals” [74]. Gomez-Carrillio et al. claim that receiving prognostic information can considerably affect individuals'self-perception. Thus “diagnostic constructs and explanations” become a reality that in a significant way influences and determines an individual's experience [75].
Lebowitz and Ahn report results of experimental studies suggesting that if people are presented with results of genetic susceptibility to depression, they tend to report having experienced more depressive symptoms in the past. They point out that this finding is particularly alarming because the current psychiatric diagnostic system very much relies on retrospective self-report of past symptoms, therefore “delivering personalized genetic information to patients may complicate the process of making psychiatric diagnoses” [76]. Therefore, prediction of risk might not only cause harm to the individuals but also impact the validity of psychiatric diagnosis as well.
Rights and responsibilities
Informed consent, personal autonomy and agency
Informed consent of patients and research participants is a constant a subject of debate in medical and research ethics. Informed consent ensures respect to autonomy, voluntariness and shared decision making if it provides necessary information, opportunities for dialog and necessary support. Nevertheless, the context of the prediction of severe mental disorders brings forward a specific set of issues. One problem is the amount and content of information that should be given to the person prior to making the risk prediction to ensure informed and voluntary consent. As Lane et al. have observed, a predicted psychiatric condition may not be well defined as “many studies include a range of different diagnoses with varied treatments and prognoses under the outcome of psychosis"and predictions are “inevitably uncertain” [36]. Prediction of risk requires that the person is provided with “clear, simple, understandable and concrete” [77] information about the purpose of risk prediction, its nature, benefits, risks, limitations, actionability and potential consequences for the individual and family. The information should be tailored to the needs and situation of a particular person. Also, it is advisable to stratify the consent, for example, by “asking them whether they want information that is clinically actionable, has reproductive implications, may affect life planning, etc” [78]. The same applies to the information about prognosis and possible benefits and disadvantages of learning one’s risk status. Lane et al. claim that there is not enough empirical research examining how individuals might react to personal risk predictions and therefore there is a lack of information on how to disclose the information in a manner that maximises autonomy [36, 74, 68, 51].
Another issue is the complexity of consent and assent in case of minors. For example, schizophrenia has a typical onset in adolescence or young adulthood [79] which means that a considerable proportion of predictive tests will be conducted when the individuals are minors, i.e., will not be at the age to have the legal capacity to consent and consent will be sought from their legal guardians [74, 80]. This raises the question of “whether and how children would be involved in the decision to be tested and in the discussion of results”? [81]. In a qualitative study by Erickson et al., most individuals with a personal and/or family history of mood disorders expressed a wish to test their children before adolescence, however, most of them also indicated that they would not share the results with the child until adulthood or until symptoms developed [81]. Cassetta et al. mention a principle that is widely accepted in many countries, namely, that if minors are barred from giving consent by law or persons are unable to consent due to their cognitive capacity, one should try to get assent for risk prediction [74]. Even in cases where individuals have reached the legal age of consent, several authors point out that testing for severe mental disorders raises questions about the capacity to consent [36, 74]. As Lane et al. observe, individuals at high risk of psychosis may have “significant levels of comorbid psychiatric disorders, intellectual disabilities, and subthreshold psychotic symptoms” [36]. That, as they note, has the potential to impair the capacity to give consent, but they also emphasise that that being the case one should not draw any generalised conclusion about the decision-making capacity of individuals with mental disorders [36] and that being at risk “does not automatically mean that an individual does not have the cognitive capacity to provide informed consent” [74] Instead, they recommend evaluating each persons’ capacity individually rather than making assumptions [74].
Rights to know and not to know
Right to know and not to know one’s genetic risk is a topic that has been widely debated in the context of testing for somatic conditions. Therefore, one of the main questions raised in the literature on genetic testing for psychiatric conditions has been, whether the psychiatric context makes any difference. Can the arguments that are used for either pro or contra rights to know and not to know one’s genetic risk regarding somatic conditions be applied in psychiatry? [65] One argument used by defenders of rights not to know one’s genetic risk regarding somatic conditions is related to actionability. Namely, if there are no treatments or preventive measures available for the condition, then the benefits of knowing might be outweighed by the harms of knowing [65]. An important dissimilarity strengthens this argument in a case of psychiatric condition. As some authors have argued, it is reasonable to consider that unlike in screening for cancer, the knowledge of being at risk of developing a psychiatric condition itself might play a causal role in developing the condition [82, 65]. Thus the case for the rights not to know is made stronger.
Privacy and confidentiality
If novel risk prediction models are being implemented in psychiatry they will likely rely on a great variety and quantity of data. Therefore, privacy, data safety and confidentiality are a concern [13, 83, 84, 36, 56]. As Manchia et al. point out: “given that precision psychiatry is strongly related to the analysis of massive datasets (either phenotypic, neuroimaging, or biological), confidentiality and privacy concerns are becoming increasingly relevant” [56]. To mitigate these concerts they suggest that “it is crucial to develop an appropriate ethical-legal framework, which would facilitate safe data sharing” [56]. In a similar vein, Fusar-Polli et al. warn about ethical concerns regarding “privacy, cybersecurity, confidentiality and device dependability” [13]. In particular, they stress that “leaking of private information can affect personal lives, including bullying, high insurance premiums, and loss of jobs due to medical and psychiatric history” [13]. They also suggest that “These concerns should be addressed by the implementation of strict data governance and security policies that comply with local regulations"[13]. Lane et al. note that empirical evidence suggests that, for example, individuals at high risk of psychosis “feel apprehensive about information security and privacy of genetic risk information"[36]. They refer to the study by Lawrence et al. which indicates that information privacy is an important concern for individuals at risk [40]. Similar concerns are expressed by participants in a study conducted by Mantell et al [33].
Another concern regarding confidentiality is related to the involvement of the family members. Cassetta and Goghari note that provided that heritability is high for several psychiatric disorders: “it is conceivable that first-degree relatives of persons who undergo screening for psychosis would have a personal interest in obtaining the results of the screening procedure” [74].
Counselling, education and communication
Need for guidelines and standards
Several authors recently point out that the lack of guidelines is an obstacle to the implementation of different aspects of prediction of risk in psychiatric clinical practice. For example, regarding novel prediction models using AI Graham et al. point out that “there are no established standards to guide the use of AI and other emerging technologies in healthcare settings” [23]. Ward et al. mention, that despite different potential applications for genetic testing in psychiatry, psychiatrists still face a “critical structural challenge”, namely “the lack of a clear standard of care and professional guidelines for the use of genomic testing and management of findings” [32]. The lack of standards creates some problems already in the research phase. As Chapman indicates in her study, in the research of polygenic scores (PGS) every research group uses different methods to develop PGS and their results therefore can diverge. She refers to a study by Docherty et al. [85] in which they conclude that the current regulatory environment enables “oversimplification and exaggeration of research results for marketing purposes” and provision of genetic tests “without demonstration of clinical validity” [43]. Ward et al. point out that provided quick development of psychiatric genomics and increasing use of testing in psychiatric practice one should expect a period of uncertainty about what constitutes a responsible use of these developing technologies. They emphasise the role of professional organisations in minimising the uncertainty by developing practice guidelines: “Such guidelines would help to establish a standard of care and would go a long way in addressing clinician concerns regarding proper practice and legal liability related to the use of genomics findings” [32].
Actionability
Most of the benefits of the clinical use of prediction of risk are dependent on the availability of clinical interventions that can either prevent or postpone the onset of severe mental illness. However, currently, these abilities are very limited and there seems to be a consensus that under such circumstances prediction of risk for a psychiatric condition is not advisable as it might cause harm without providing any clinical benefits [86]. Empirical data indicates that individuals and families who are interested in testing at least partly share this attitude. Namely, as Meiser et al. report in their review, adults’ interest in genetic testing for psychiatric conditions depends not only on the predictive value of the test but also on the availability of treatment and/or prevention options [39]. However, there is evidence that some individuals would be interested in testing notwithstanding treatment possibilities [63]. Parens et al. find that a surprising result [87], but this might point to the importance of non-clinical benefits that individuals expect from testing. Moreover, it seems that the preventive measures should not be understood too narrowly. This point is emphasised by Fusar Poli et al. According to them, one might object that “if no effective preventive intervention can be provided, then knowing in advance may not be helpful outside clinical monitoring (which can reduce the duration of untreated disorder). However, young people accessing preventive (e.g., CHR-P) services benefit from an integrated package of vocational, psychosocial and familial support interventions which would otherwise not be available to them” [88].
Education of professionals and laypeople
For the successful implementation of prediction models in clinical practice, some basic knowledge of the technologies involved is essential for all the stakeholders, and even in-depth knowledge might be needed for professionals using them in clinical practice. However, several studies indicate that there are no good reasons to be optimistic in this regard. The study by Erickson and Cho suggests that the general public lacks understanding and education “surrounding the field of genetics” [64]. They point out that “The majority of researchers were in agreement that not only is there a lack of genetic knowledge among members of the general public, but there is also great difficulty in understanding probability and risk” [64]. This is an important issue, because in that case “even if someone is given information about his/her genetic risk for a psychiatric disorder, it is likely the results will either be meaningless or misunderstood by the patient"[64]. Most likely the same applies to other fields involved in the novel risk prediction models, like, epigenetics and neuroimaging, as well as to the use of AI. Fusar-Poli et al. also mention this as an ethical challenge that should be dealt with [13]. However, it seems that similar concerns apply to health providers as well. The experts in psychiatric research interviewed by Erickson and Cho “emphasised that this lack of genetic risk education is a problem not only among the lay population. Almost every genetic researcher interviewed (n = 25) strongly believed that the majority of physicians and health care providers do not have the adequate knowledge or experience to help patients interpret genetic test results or to put these results in the correct context of probability or risk"[64]. Bloss et al. also mention the physicians’ lack of training and knowledge of genomics as a hindrance to adopting genetic testing in clinical practice [89]. Use of AI or use and interpretation of epigenetic or neuroimaging data may pose similar challenges. Fusar-Poli et al. point out that what matters in the communication of risks is not only knowledge but also the methods of disclosing:"the ability to communicate the results of a risk prediction analysis ethically relies heavily on the competence, level of knowledge and training, and skills of the health professionals. [..] This appears particularly crucial for sharing behavioural genetics findings [..], given that the risk of misinterpreting results might increase the potential for discrimination and stigma"[13]. Lack of the knowledge and understanding may become a serious obstacle also for obtaining a voluntary and fully informed consent.
Counselling and empowerment
As discussed above, individuals'and healthcare professionals’ lack of genetic literacy and other types of knowledge underscore the importance of counselling on results of risk prediction. As Appelbaum and Benston claim: “Optimal genetic counselling incorporates both education and encouragement, assisting patients in embracing their autonomy and understanding what sort of control they might have in managing their symptoms"[49]. To underscore the importance of counselling, they refer to an empirical study by Inglis et al. [90] which shows that specialised genetic psychiatric counselling increases individuals’ sense of empowerment and self-efficacy. Lawrence et al. have conducted an empirical interview-based study with young adults at clinical high risk of psychosis and claim that their findings “validate the important role of genetic counsellors, who are well positioned to help persons understand the risks and benefits of getting tested, understand their test results, and decide whom to tell"[40]. Evidence for the benefits of psychiatric genetic counselling is also provided by the study of Costain et al. [91] and described by Mundy et al. [92] However, Mundy et al. point to an important problem, namely, that the majority of the empirical studies are aimed at the adult population. Provided that the novel prediction models for familial risk might be used in children and adolescents, this is a relevant gap that should be addressed by researchers. Further, as Appelbaum and Benston observe, the role of counsellors in psychiatry has been rather limited up till now. Part of the reason for this is the small number of them, at least in the U.S. Further, they point out that many of the existing counsellors know little about psychiatric disorders [49]. Mundy et al. point out that the number of genetic counsellors falls below the recommendations by the WHO. They mention that one strategy to reduce the reliance on counsellors might be to train other healthcare professionals the necessary skills and there is some empirical evidence that might support such a move, however, more empirical research is needed to study that [92].
Ethical issues in different applications
Reproductive choices and prenatal use
Prediction of risk for severe mental disorders might affect the reproductive choices that people make. Hoge and Appelbaum note that “Preliminary studies suggest there is substantial interest in prenatal testing for other neuropsychiatric disorders such as bipolar disorder [..], schizophrenia, alcoholism, attention deficit disorder and depression” [78]. They suggest that the high level of interest in testing "may reflect a lack of understanding of the likely value of predictive testing"[78]. Laegsgaard and Mors surveyed the attitudes of patients with psychiatric diagnosis, their relatives and medical and psychology students and report that the support for prenatal testing is weaker than for testing of children or self [41]. Moreover, the participants showed less support for prenatal testing for psychiatric than for somatic illness. They point out that similar results are reported in a study conducted by Smith et al. [93] in which they measured the intentions of bipolar patients, medical students and psychiatric students regarding termination of pregnancy in a case of a positive predictive genetic test result. The proportion of those participants who would terminate pregnancy for risk of severe life-threatening disease was higher than those who would terminate pregnancy for risk of bipolar disorder. Smith et al. think that this result can be explained by the fact that somatic diseases are perceived as potentially more fatal than bipolar disorder [93]. A recent systematic review by Meiser et al. paints a similar picture. The studies included in their review report that attitudes towards prenatal testing were less positive than those to testing of adults or children. Further, “The observed trends indicate that the decision to terminate a pregnancy may be influenced by the likelihood of the condition as well as the anticipated severity of the disorder"[39]. The reasons mentioned for the lack of support for prenatal testing were: “being against pregnancy termination in general, not considering the condition (e.g., bipolar disorder) as serious enough to warrant termination, not considering termination because of the increasing number of treatments that are becoming available, and the view that prenatal testing and selective termination could deprive humanity of the positive effects of bipolar disorder, such as increased productivity and creativity"[39]. Some authors have expressed concerns that prenatal testing for psychiatric conditions would be conducted for eugenic purposes [64, 94, 42, 95].
Use in minors
Hoge and Appelbaum have raised the concern that “Parents may seek to have their asymptomatic children tested for susceptibility to disorders that might appear later in life, invoking their right to know their children’s vulnerabilities and to make family decisions"[78]. They argue that this would go against children’s interests as they would not get any immediate benefit and in addition to that their “choice whether to be tested later in life will be pre-empted” [78]. Meiser et al. in their review mention that the studies conducted to assess attitudes towards predictive psychiatric genetic testing for children show that interest in testing ranges from 61 to 83% [39]. Not surprisingly, interest among patients was stronger than interest among at-risk relatives. The main concerns raised by testing children are the potential harms that testing can bring. The participants of the studies reviewed by Meiser et al. have expressed the concern that knowing about a child’s risk will cause anxiety. Some participants feared that the knowledge alters parents'behaviour towards their children and contributes to “child’s risk of developing psychopathology in the future"[39]. Hoge and Appelbaum speculate that parents of a child identified as being at risk may allocate resources for education to their other children or try to protect the child thus affecting its development or self-image and that the test results may lead to discrimination [78]. Morris and Heinssen point out that adolescents"may be impacted by greater responsiveness to peer pressure, altered risk perception, and increased focus on short-term risks and benefits” [80]. As a consequence “these tendencies can affect adolescents’ thinking about their risk of developing illness as well as their risk of experiencing discomfort or adverse outcomes related to participation in a research study or clinical intervention"[80]. Lane et al. mention the view shared by several authors that “In the field of clinical genetics, predictive testing of minors for adult-onset conditions is contra-indicated unless interventions in childhood can prevent or ameliorate illness"[36]. They point out that the ethical reasoning used to justify this view relies on the respect of children’s future autonomy and the concept of the right to an open future. Lane et al. claim that “In the case of psychosis prediction it is uncertain whether the therapeutic gain from pre-emptive interventions in childhood or adolescence is compelling enough to justify restricting future autonomy in this manner” [36]. Manzini and Vears argue against this view. They claim that the argument about future autonomy presumes that “individuals suddenly become self-determinant when they reach the age of majority (often 18 years)"[35]. Minors, they argue, do not constitute a homogenous group. Children develop and that means that"some individuals below the age of majority have the intellectual capacity and the emotional maturity to make competent decisions themselves” [35]. Besides this, they also argue that the benefits of testing in minors should not be conceptualised too narrowly and there is some evidence that indicates that testing may reduce anxiety and have positive effects on family relationships. Regarding the use of prediction of risk for psychiatric conditions, it could be relevant to take into account the point of view of minors themselves. Empirical studies conducted on this topic indicate that minors are interested in predictive testing. The study conducted by Pavarini et al. involving 80 UK adolescents aged 16–18 years, found that"most participants were interested in taking a mental health predictive test, especially for anxiety, learning difficulties and depression"[96]. They point out that their results align with other studies"showing adolescents value learning about themselves and their health” [96]. The participants were specifically motivated to take the tests if they saw that the information provided by the tests was relevant to them due to their family conditions or symptomatology. This, as Pavarini et al. claim, “is supported by prior research that documented high interest in learning about one’s risk among individuals at clinical high risk for psychosis and those with familial experience of depression"[96].
Family and relatives
An ethical issue that is discussed regarding prediction of risk in the context of family is whether there is a duty to share the results of testing with other members of the family. The question has previously been broadly discussed in the context of testing for somatic conditions and whether testing for psychiatric conditions can be treated in the same way. Appelbaum claims that concerning testing for somatic disorders the duty to warn a relative about the test result is conditional on three factors: “1) the likelihood that family members are at risk; 2) the severity of the potential consequences; and 3) whether effective interventions exist to mitigate the risk"[97]. He argues that it will be much harder to justify a duty to warn in case of genetic testing for psychiatric disorders because “the presence of one or more alleles associated with a disorder is likely to lead to only a modest absolute risk, the disorder itself will be treatable to some considerable extent, and preventive measures are likely not to be available” [97]. DeLisi also points out that the duty to inform is much less clear in the case of risk factors of schizophrenia as the risk of developing the condition for relatives is, although increased still rather low [48]. Daws concurs, saying that “Clinically, the duty to inform is made less potent by schizophrenia’s unique association with cognitive ability, which should be factored into deciding whether or not to apprise a patient of their risk"[55]. Appelbaum suggests that a “more reasonable and less intrusive” way of action would be to advise the patient on the possible risks to his or her family members [97]. At the same time, there is evidence that a considerable proportion of individuals actually share their test results with their close ones. Meiser et al. refer to a study by Potokar et al. [98] according to which “the majority of people (78%) with serious mental illness would share their genetic testing result with family and friends” [39]. Another study they mention [40] involved individuals at high risk of schizophrenia considering hypothetical genetic testing. According to the results, the participants “would tell people with whom they felt comfortable and whom they trusted about their genetic risk"[39].
Research ethics
As novel tools for predicting mental illness risk are still under development, the review highlights specific ethical and social issues in the research context. A research study begins with selecting the research topic, a step that inherently ethical considerations. Then it is followed by recruitment of research participants, and the researchers have to ensure that the potential research participants fully grasp the implications of participation—in this case implications of testing mental health risks.. In a 2004 paper on ethical issues in psychiatric genetics Appelbaum raises the question ‘what should research participants be told about the results of the tests they undergo?’ bearing in mind that, e.g.,"[m]ost of the purported associations of genetic loci with psychiatric disorders have subsequently been disproved” and in the context of genetics absolute risk from a single allele is expected to be minimal [97]. Around the same time, Biesecker and Peay discuss the importance of genetic counselling for research participants who are receiving genetic test results on the risk of psychiatric illness [99]. A decade later Bieseker returns to the topic and emphasises that “An important aspect of consenting to these studies is an appreciation for the degree of uncertainty”, including the risk of false positive and false negative research results [100].
Another question is how to deal with incidental and secondary findings that are expected to emerge in the context of research due to the extensive genetic and other types of data analysed [50].
Discussion
Our analysis revealed four main themes that have been discussed in the literature thus far: (1) potential harms and benefits, (2) rights and responsibilities, (3) counselling, education and communication, and (4) ethical issues in different applications. Each of the themes include numerous subtopics. In what follows, we comment on some frequently discussed subtopics and then move to the topics that were less prominent in the literature.
The rationale for developing tools to predict individual risk of developing a severe mental disorder is the benefits that such tools bring to individuals at risk and their families. In the literature, great emphasis is put on the benefits that are directly clinical, e.g., predicting risks early allows for preventive (risk reduction or resilience strengthening) measures, thereby reducing the likelihood or delaying the onset of a disease. At the same time, many authors point towards the benefits that are not (at least directly) clinical or medical, e.g., that predictive information can positively affect an individual’s behaviour, give individuals and their family members a feeling of control, reduce anxiety, improve family relationships, etc. However, because it is very difficult or even impossible to measure or quantify these positive effects, making a cost-benefits analysis of the clinical use of prediction tools is problematic. The empirical evidence shows that the individuals’ hypothetical interest in risk prediction is rather high which counts as an argument to offer it. However, would this hypothetical interest translate into real-life uptake of the risk prediction? It is, of course, impossible to predict, but if predictive genetic testing for somatic disorders can provide any guidance then the answer most likely is more negative than positive. As Laegsgard and Mors and others point out, empirical evidence indicates that hypothetical interest in predictive genetic testing for somatic conditions as Huntington's disease and cancer is a poor predictor of actual test uptake [41, 101].
It is potentially useful to make a distinction between families with or without high in this case. According to the findings reported above [96] the uptake of testing is facilitated by family history. It is reasonable to speculate then that at risk families will be more motivated to use the predictive tools than families who are not at risk. This claim however should be backed by further evidence.
There are other factors that may facilitate the uptake of testing. In a recent systematic review of literature on the predictors of genetic testing decisions both for somatic and psychiatric disorders Sweeny et al. conclude that “people are more likely to test when they perceive many benefits of testing, few barriers to and risks of testing, and positive attitudes surrounding testing” [102]. In comparison, genetic disorder-related variables as perceived risk, perceived control and severity showed much weaker impact on the decision being tested [102]. This underscores the importance of highlighting the benefits that prediction of risk might provide.
The eventual cost–benefit ratio of clinical use of novel predictive tools in psychiatry will be determined by the number and weight of the possible risks, harms and concerns that such risk prediction might bring relative to the benefits. And the concerns described in the literature are numerous. First, results can be false negative (no or low risk is wrongly communicated) or false positive (high risk is wrongly communicated). Second, being said to be at risk brings harm. It might negatively affect an individual’s behaviour, cause anxiety, change the person’s self-conception, people might adopt a patient’s identity and that might further exacerbate their condition, the prediction itself might serve as a trigger event for the condition in question, etc. Third, there are a number of concerns that are related to the social context, e.g., if disclosed, prediction of risk could affect an individual's job opportunities or give rise to discrimination, negative labelling or stigmatisation.
Fourth, a concern that has attracted considerable attention in the literature is stigmatisation. To some extent, it is certainly justified as stigmatisation is one of the most often discussed issues regarding mental disorders in general and numerous empirical studies indicate that the phenomenon is real and significant. But it is also complex. There are at least two reasons why the concerns of stigmatisation should be discussed with particular care.
First, there are two opposing strands. An optimistic line of reasoning argues that a genetic explanation of psychiatric disorders decreases the stigmatisation of individuals with psychiatric disorders, while a pessimistic one argues for the opposite view, namely, that it increases stigmatisation. There is a simple and empirically based explanation for this contradiction. In a meta-analytic review of associations between biogenetic explanation of mental disorders and three key elements of stigma (blame, perceptions of dangerousness, social distance) Kvaale et al. conclude that individuals who rely on biogenetic explanations of mental disorders are less likely to blame affected individuals for their issues, however, they perceive them as more dangerous and want to distance themselves from them [103, 104]. Therefore, biogenetic explanations of psychiatric disorders have “mixed blessings” [105]. Based on this work, one could speculate that clinical application of novel risk prediction models for severe mental disorders also has differential effects on stigmatisation.
Second, studies on stigmatisation usually address stigma towards individuals with mental disorders, and there are also some studies on stigmatisation of individuals with prodromal or subsyndromal psychotic symptoms [106], but evidence is lacking on whether stigma is experienced in similar way in asymptomatic individuals at risk. Interestingly, a recent experimental vignette-based study involving health care professionals [107] suggested that concerns about the increase of stigmatisation due to the use of predictive tools are justified. The results they report generally correspond to the conclusions reached by Kvaale et al. [103, 104]. That is, they found that novel predictive tools for psychiatric disorders might decrease some aspects of stigma (e.g., anger), but increase others (e.g., fear).
Further, there are more possible negative effects of receiving prognostic information about mental health risk. Gomez-Carillo et al. refer to some studies that show how perceived risk affects individuals even more than the actual genetic risk. They write “Similarly, receiving a diagnosis that conveys a specific prognosis can affect the course of illness and treatment response, in part through placebo or nocebo responses and other broader expectancy responses [..] as well as potentially leading to social stigma with consequences for self-efficacy, help-seeking, and employment status."[75] Although they talk about diagnosis and prediction of prognosis here, it seems that the same applies to prediction of diagnosis as well. To explain this point, they involve the concept introduced by philosopher Ian Hacking—“the looping effect of human kinds” which refers to the feedback effect when the meanings of science classifications affect the behaviour of people who fall under that classification [108, 109]. They suggest that in case of a psychiatric disorder, there is a “process of “bio-looping” in which cognitive, behavioural, and interpersonal processes feedback to alter the individual’s neurobiology in addition to their social and psychological effects” [75].
The literature thus far does not adequately address issues related to the employment of machine learning and other AI algorithms in predictive tasks. This is surprising, given the important role this technology plays in novel prediction tools. However, the technology is still relatively novel. One of the concerns that is raised is unexplainability or opacity. As Lane and Broome put it: “Current personalised prediction models often lack transparency in terms of not supplying the user with an explanation of how or why a particular risk estimate is made. In addition, the complexity of statistical and machine learning methods makes it extremely difficult (and often impossible) [..] to decipher the prediction model’s workings"[8]. Henceforth, the claim is that so-called “black-box” machine learning models should be avoided. Is such a strict view justified? The ethical principle of explicability regarding AI was formulated by Floridi et al. in the programmatic report of the AI4People Scientific Community which attempts to lay out the foundations for a “Good AI Society” [110]. To accomplish that, they use the framework of four principles (autonomy, beneficence, non-maleficence, justice) developed by T. L. Beauchamp and J. F. Childress [111] and widely adopted in biomedical ethics by adding one more principle—explicability. The principle of explicability according to Floridi et al. includes two requirements—“the need to understand and hold to account the decision-making processes of AI” [110]. However, first, some authors have challenged the view that explicability should be treated as a separate principle in biomedical ethics [112, 113]. Further, in bioethics literature on ethical issues of using machine learning in medicine, there is no consensus on how strict the requirement of explicability is. For example, Wang et al. point out that black-box models are not that different from other areas in medicine where health-care professionals lack complete biological or clinical knowledge: “Clinicians cannot always explain why they arrived at a particular diagnosis. Many effective drugs—were in widespread use for decades before their mechanism or action was understood. It is still not clear how electroconvulsive therapy or selective serotonin reuptake inhibitors work” [114]. It seems that it would be wrong then to hold AI to a higher standard than drugs or different types of medical devices. No doubt, the clinical use of machine learning algorithms raises new issues that should be addressed [115, 116], but as Friedrich et al. argue “Explainability need not be the determining factor when deciding whether to deploy black-box AI in medicine. Rather, physicians ought to be able to use their own clinical reasoning, combined with patient values and preferences, to use black-box AI—and all technologies—prudently” [117]. To find out whether the lack of explainability of machine learning algorithms is a real obstacle, it would be important to find out—as far as it is possible in the circumstances—how all the stakeholders (e.g. potential users of prediction tools, their family members, clinical psychiatrists and genetic counsellors) view the issue. Especially, how important explicability for them is in the context of other factors, such as prognostic accuracy, risk of bias, availability, uptake, etc. As with many other concerns, this area would certainly benefit from insights gathered via empirical research. Further, one should take into account that being able to explain at the level of the individual which factors drive or reduce the risk is relevant treatment decisions. This information potentially gives insight in the type of prevention an individual will benefit most from (e.g. trauma therapy or social skills training) thereby guiding treatment decisions.
There are two other issues that would deserve more attention than received so far in the literature—the clinical utility and concerns about excessive or mandatory use. Clinical utility, cost–benefit ratio and availability are key elements for the implementation of novel prediction tools in clinical practice. How likely is it that use of novel prediction tools will result in an improved health outcome? What will be the costs of the method? How available will it be to the individuals who need it? These and similar questions seem especially pertinent considering how underfunded mental health care is in many countries [118].
Concerns about excessive or mandatory use of prediction tools are only addressed by Manzini and Vears [35] stating that as soon as genetic screening becomes an acceptable practice this may raise societal pressure on parents to screen their children for psychiatric disorders and therefore undermine parental decision-making authority. To counter this objection, they point to the complexity of gene-environment interaction and that one of the benefits of genetic testing is that it enables parents to pursue early screening, thereby giving an opportunity to reduce the exposure of the child, who is at risk, to environmental risk factors. If that is the case the genetic testing in fact enhances parental decision-making autonomy. This is an interesting move, but their opponents might still insist that the main concern is not resolved as the pressure might be not only societal, it might also be institutional, e.g., in government-funded healthcare systems, some healthcare benefits might be made conditional on the testing.
Although we tried to provide as comprehensive a picture as possible, the review has some limitations that should be mentioned. First, only English language articles were included.
Second, most of the articles were looking at genetic testing of psychiatric diseases and did not include analysis of prediction tools integrating several biomarkers and AI. This is relevant because there are many different approaches to risk prediction—an app in the hands of users, a tool for physicians or a tool that is only used by a genetic counsellor. Specific forms of implementation come with their own risks and benefits, which are not addressed in this study.
Conclusions
The review reveals a wide range of ethical and societal concerns that should be taken into account in developing and implementing novel prediction tools in clinical practice. Our review makes clear that some issues have received more attention (e.g. stigmatisation) than others (excessive or mandatory use, AI ethics), pointing towards several gaps that should be addressed in future, preferably empirical, research. Further, to obtain a more inclusive picture it is essential to research the views of all the relevant stakeholders involved—individuals and families at risk, researchers, (child and adolescent) psychiatrists and other mental health care workers. Moreover, it is reasonable to assume that existing differences in mental health care systems between countries and cultural differences affect how stakeholders view risk prediction. Therefore, researchers should be careful when they extrapolate their empirical findings to populations that are not included in their studies.
Finally, we found indications that social and ethical concerns weigh differently in families with high risk than in the general population. Currently, however, there is not much evidence to substantiate this claim with empirical data and further research is needed. The FAMILY project with its focus on the family context aims to contribute in mitigating this gap.
Data availability
The authors confirm that all data generated or analyzed during this study are fully available in this published article and its supplementary files.
Abbreviations
- AI:
-
Artificial intelligence
- PRISMA-ScR:
-
Systematic Reviews and Meta-Analyses Extension for Scoping Reviews
References
Uher R, Pavlova B, Radua J, Provenzani U, Najafi S, Fortea L, et al. Transdiagnostic risk of mental disorders in offspring of affected parents: a meta-analysis of family high-risk and registry studies. World Psychiatry. 2023;22:433–48.
Rasic D, Hajek T, Alda M, Uher R. Risk of mental illness in offspring of parents with schizophrenia, bipolar disorder, and major depressive disorder: a meta-analysis of family high-risk studies. schizophr bull. 2014;40:28–38.
Maciejewski D, Hillegers M, Penninx B. Offspring of parents with mood disorders: time for more transgenerational research, screening and preventive intervention for this high-risk population. Curr Opin Psychiatry. 2018;31:349.
Harries CI, Smith DM, Gregg L, Wittkowski A. Parenting and Serious Mental Illness (SMI): a systematic review and metasynthesis. Clin Child Fam Psychol Rev. 2023;26:303–42.
Duffy A, Goodday SM, Christiansen H, Patton G, Thorup AAE, Preisig M, et al. The well-being of children at familial risk of severe mental illness: an overlooked yet crucial prevention and early intervention opportunity. Nat Ment Health. 2023;1:534–41.
Raballo A, Poletti M, Preti A. Applying transgenerational scientific evidence to the next wave of early identification strategies for psychopathological risk—transdiagnostic, developmental, and personalized. JAMA Psychiat. 2021;78:1067–8.
Salazar de Pablo G, Studerus E, Vaquerizo-Serrano J, Irving J, Catalan A, Oliver D, et al. Implementing precision psychiatry: a systematic review of individualized prediction models for clinical practice. schizophr bull. 2021;47:284–97.
Lane N, Broome M. Towards personalised predictive psychiatry in clinical practice: an ethical perspective. Br J Psychiatry. 2022;220:172–4.
Rosen M, Betz LT, Schultze-Lutter F, Chisholm K, Haidl TK, Kambeitz-Ilankovic L, et al. Towards clinical application of prediction models for transition to psychosis: a systematic review and external validation study in the PRONIA sample. Neurosci Biobehav Rev. 2021;125:478–92.
Sanfelici R, Dwyer DB, Antonucci LA, Koutsouleris N. Individualized diagnostic and prognostic models for patients with psychosis risk syndromes: a meta-analytic view on the state of the art. Biol Psychiatry. 2020;88:349–60.
Hafeman DM, Uher R, Merranko J, Zwicker A, Goldstein B, Goldstein TR, et al. Person-level contributions of bipolar polygenic risk score to the prediction of new-onset bipolar disorder in at-risk offspring. J Affect Disord. 2025;368:359–65.
van Houtum LAEM, Baaré WFC, Beckmann CF, Castro-Fornieles J, Cecil CAM, Dittrich J, et al. Running in the FAMILY: understanding and predicting the intergenerational transmission of mental illness. Eur Child Adolesc Psychiatry. 2024. https://doiorg.publicaciones.saludcastillayleon.es/10.1007/s00787-024-02423-9.
Fusar-Poli P, Manchia M, Koutsouleris N, Leslie D, Woopen C, Calkins ME, et al. Ethical considerations for precision psychiatry: a roadmap for research and clinical practice. Eur Neuropsychopharmacol. 2022;63:17–34.
Tricco AC, Lillie E, Zarin W, O’Brien K, Colquhoun H, Kastner M, et al. A scoping review on the conduct and reporting of scoping reviews. BMC Med Res Methodol. 2016;16:15.
Parsons JA, Johal HK. In defence of the bioethics scoping review: largely systematic literature reviewing with broad utility. Bioethics. 2022;36:423–33.
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169:467–73.
Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc. 2016;104:240–3.
Gibbs GR. Analyzing Qualitative Data. London: SAGE Publications Ltd; 2018.
Aria M, Cuccurullo C. bibliometrix: an R-tool for comprehensive science mapping analysis. J Informetr. 2017;11:959–75.
van Eck NJ, Waltman L. Visualizing Bibliometric Networks. In: Ding Y, Rousseau R, Wolfram D, editors. Measuring scholarly impact: methods and practice. Cham: Springer International Publishing; 2014. p. 285–320.
Davidson M. Can premorbid and prodromal markers associated with psychosis be utilized for early detection and secondary prevention of schizophrenia? Dialogues Clin Neurosci. 2001;3:138–43.
Marriott CAL, Broome MR. The ethics of early identification and intervention in psychosis. In: Cratsley K, Radden J, editors. Mental health as public health: interdisciplinary perspectives on the ethics of prevention. Elsevier; 2019.
Graham S, Depp C, Lee EE, Nebeker C, Tu X, Kim HC, et al. Artificial intelligence for mental health and mental illnesses: an overview. Curr Psychiatry Rep. 2019;21:116.
Chekroud AM, Bondar J, Delgadillo J, Doherty G, Wasil A, Fokkema M, et al. The promise of machine learning in predicting treatment outcomes in psychiatry. World Psychiatry. 2021;20:154–70.
Galbraith Q, Butterfield AC, Cardon C. Judging journals: how impact factor and other metrics differ across disciplines. Coll Res Libr. 2023;84:888.
Weinberg BH. Bibliographic coupling: a review. Inf Storage Retr. 1974;10:189–96.
Kostick K, Pereira S, Brannan C, Torgerson L, Lazaro-Muñoz G. Psychiatric genomics researchers’ perspectives on best practices for returning results to individual participants. Genet Med. 2020;22:345–52.
Kostick KM, Brannan C, Pereira S, Lazaro-Muñoz G. Psychiatric genetics researchers’ views on offering return of results to individual participants. Am J Med Genet B Neuropsychiatr Genet. 2019;180:589–600.
Lázaro-Muñoz G, Sabatello M, Huckins L, Peay H, Degenhardt F, Meiser B, et al. International society of psychiatric genetics ethics committee: issues facing us. Am J Med Genet B Neuropsychiatr Genet. 2019;180:543–54.
Lázaro-Muñoz G, Torgerson L, Pereira S. Return of results in a global survey of psychiatric genetics researchers: practices, attitudes, and knowledge. Genet Med. 2021;23:298–305.
Pereira S, Muñoz KA, Small BJ, Soda T, Torgerson LN, Sanchez CE, et al. Psychiatric polygenic risk scores: child and adolescent psychiatrists’ knowledge, attitudes, and experiences. Am J Med Genet B Neuropsychiatr Genet. 2022;189:293–302.
Ward ET, Kostick KM, Lázaro-Muñoz G. Integrating genomics into psychiatric practice: ethical and legal challenges for clinicians. Harv Rev Psychiatry. 2019;27:53.
Mantell PK, Baumeister A, Ruhrmann S, Janhsen A, Woopen C. Attitudes towards risk prediction in a help seeking population of early detection centers for mental disorders-a qualitative approach. Int J Environ Res Public Health. 2021;18:1036.
Leopold K, Ritter P, Correll CU, Marx C, Ozgurdal S, Juckel G, et al. Risk constellations prior to the development of bipolar disorders: rationale of a new risk assessment tool. J Affect Disord. 2012;136:1000–10.
Manzini A, Vears DF. Predictive psychiatric genetic testing in minors: an exploration of the non-medical benefits. J Bioethical Inq. 2018;15:111–20.
Lane NM, Hunter SA, Lawrie SM. The benefit of foresight? An ethical evaluation of predictive testing for psychosis in clinical practice. NeuroImage Clin. 2020;26:102228.
Heinssen RK, Perkins DO, Appelbaum PS, Fenton WS. Informed consent in early psychosis research: National Institute of Mental Health workshop, November 15, 2000. Schizophr Bull. 2001;27:571–83.
Erickson JA, Cho MK. Interest, rationale, and potential clinical applications of genetic testing for mood disorders: a survey of stakeholders. J Affect Disord. 2013;145:240–5.
Meiser B, Guo XY, Putt S, Fullerton JM, Schofield PR, Mitchell PB, et al. Psychosocial implications of living with familial risk of a psychiatric disorder and attitudes to psychiatric genetic testing: a systematic review of the literature. Am J Med Genet B Neuropsychiatr Genet. 2020;183:277–88.
Lawrence RE, Friesen P, Brucato G, Girgis RR, Dixon L. Concerns about genetic testing for schizophrenia among young adults at clinical high risk for psychosis. AJOB Empir Bioeth. 2016;7:193–8.
Laegsgaard MM, Mors O. Psychiatric genetic testing: Attitudes and intentions among future users and providers. Am J Med Genet B Neuropsychiatr Genet. 2008;147:375–84.
Palk AC, Dalvie S, De Vries J, Martin AR, Stein DJ. Potential use of clinical polygenic risk scores in psychiatry - Ethical implications and communicating high polygenic risk. Philos Ethics Humanit Med. 2019;14:4.
Chapman CR. Ethical, legal, and social implications of genetic risk prediction for multifactorial disease: a narrative review identifying concerns about interpretation and use of polygenic scores. J Community Genet. 2023;14:441–52.
Walsh CG, Chaudhry B, Dua P, Goodman KW, Kaplan B, Kavuluru R, et al. Stigma, biomarkers, and algorithmic bias: recommendations for precision behavioral health with artificial intelligence. JAMIA Open. 2020;3:9–15.
Corcoran C, Malaspina D, Hercher L. Prodromal interventions for schizophrenia vulnerability: the risks of being “at risk.” Schizophr Res. 2005;73:173–84.
Heinimaa M, Larsen TK. Psychosis: conceptual and ethical aspects of early diagnosis and intervention. Curr Opin Psychiatry. 2002;15:533–41.
Lawrie SM, Fletcher-Watson S, Whalley HC, McIntosh AM. Predicting major mental illness: ethical and practical considerations. BJPsych Open. 2019;5:e30.
DeLisi LE. Ethical issues in the use of genetic testing of patients with schizophrenia and their families. Curr Opin Psychiatry. 2014;27:191–6.
Appelbaum PS, Benston S. Anticipating the ethical challenges of psychiatric genetic testing. Curr Psychiatry Rep. 2017;19:39.
Ahlgrim NS, Garza K, Hoffman C, Rommelfanger KS. Prodromes and preclinical detection of brain diseases: surveying the ethical landscape of predicting brain health. eNeuro. 2019;6:ENEURO.0439-18.2019.
Martinez-Martin N, Dunn LB, Roberts LW. Is it ethical to use prognostic estimates from machine learning to treat psychosis? AMA J Ethics. 2018;20:804–11.
Smith WR, Appelbaum PS, Lebowitz MS, Gülöksüz S, Calkins ME, Kohler CG, et al. The ethics of risk prediction for psychosis and suicide attempt in youth mental health. J Pediatr. 2023;263:113583.
Starke G, Clercq ED, Borgwardt S, Elger BS. Computing schizophrenia: ethical challenges for machine learning in psychiatry. Psychol Med. 2021;51:2515–21.
Dwyer D, Koutsouleris N. Annual research review: translational machine learning for child and adolescent psychiatry. J Child Psychol Psychiatry. 2022;63:421–43.
Daws S. Ethical application of precision medicine to schizophrenia management. New Bioeth. 2017;23:147–53.
Manchia M, Pisanu C, Squassina A, Carpiniello B. Challenges and future prospects of precision medicine in psychiatry. Pharmacogenomics Pers Med. 2020;13:127–40.
Tairyan K, Illes J. Imaging genetics and the power of combined technologies: a perspective from neuroethics. Neuroscience. 2009;164:7–15.
Kong C, Dunn M, Parker M. Psychiatric genomics and mental health treatment: setting the ethical agenda. Am J Bioeth. 2017;17:3–12.
Ruhrmann S, Klosterkotter J, Bodatsch M, Nikolaides A, Julkowski D, Hilboll D, et al. Chances and risks of predicting psychosis. Eur Arch Psychiatry Clin Neurosci. 2012;262:S85-90.
Brannan C, Foulkes AL, Lazaro-Muñoz G. Preventing discrimination based on psychiatric risk biomarkers. Am J Med Genet B Neuropsychiatr Genet. 2019;180:159–71.
Bunnik EM, Schermer MH, Janssens ACJW. The role of disease characteristics in the ethical debate on personal genome testing. BMC Med Genomics. 2012;5:4.
Moritz S, Andresen B, Sengutta M. The specificity of schizotypal scales and some implications for clinical high-risk research. Personal Individ Differ. 2019;151:109450.
Laegsgaard MM, Stamp AS, Hall EOC, Mors O. The perceived and predicted implications of psychiatric genetic knowledge among persons with multiple cases of depression in the family. ACTA Psychiatr Scand. 2010;122:470–80.
Erickson J, Cho M. Ethical considerations and risks in psychiatric genetics: preliminary findings of a study on psychiatric genetic researchers. AJOB Prim Res. 2011;2:52–60.
Bortolotti L, Widdows H. The right not to know: the case of psychiatric disorders. J Med Ethics. 2011;37:673–6.
Mittal VA, Dean DJ, Mittal J, Saks ER. Ethical, legal, and clinical considerations when disclosing a high-risk syndrome for psychosis. Bioethics. 2015;29:543–56.
Putt S, Yanes T, Meiser B, Kaur R, Fullerton JM, Barlow-Stewart K, et al. Exploration of experiences with and understanding of polygenic risk scores for bipolar disorder. J Affect Disord. 2020;265:342–50.
Ferioli E, Picozzi M. Ethical implications of genetic susceptibility testing: NeuroGenEthics and the “Angelina Jolie effect.” Med Hist. 2018;2:166–73.
Nwulia EA, Hipolito MM, Aamir S, Lawson WB, Nurnberger JI Jr, BiGS, et al. Ethnic disparities in the perception of ethical risks from psychiatric genetic studies. Am J Med Genet B Neuropsychiatr Genet. 2011;156:569–80.
Fricker M. Epistemic injustice: power and the ethics of knowing. Oxford: Oxford University Press; 2007.
Crichton P, Carel H, Kidd IJ. Epistemic injustice in psychiatry. BJPsych Bull. 2017;41:65–70.
Mitchell PB, Meiser B, Wilde A, Fullerton J, Donald J, Wilhelm K, et al. Predictive and diagnostic genetic testing in psychiatry. Clin Lab Med. 2010;30:829–46.
Wilde A, Meiser B, Mitchell PB, Schofield PR. Public interest in predictive genetic testing, including direct-to-consumer testing, for susceptibility to major depression: preliminary findings. Eur J Hum Genet. 2010;18:47–51.
Cassetta BD, Goghari VM. Ethical considerations of screening and early intervention for clinical high-risk psychosis. ETHICS Behav. 2015;25:1–20.
Gómez-Carrillo A, Paquin V, Dumas G, Kirmayer LJ. Restoring the missing person to personalized medicine and precision psychiatry. Front Neurosci. 2023;17:1041433.
Lebowitz MS, Ahn WK. Testing positive for a genetic predisposition to depression magnifies retrospective memory for depressive symptoms. J Consult Clin Psychol. 2017;85:1052–63.
Quattrocchi A, Del Fante Z, Di Fazio N, Romano S, Volonnino G, Fazio V, et al. Personalized medicine in psychiatric disorders: prevention and bioethical questions. Clin Ter. 2019;170:E421–4.
Hoge SK, Appelbaum PS. Ethics and neuropsychiatric genetics: a review of major issues. Int J Neuropsychopharmacol. 2012;15:1547–57.
Walker EF. Adolescent neurodevelopment and psychopathology. Curr Dir Psychol Sci. 2002;11:24–8.
Morris SE, Heinssen RK. Informed consent in the psychosis prodrome: ethical, procedural and cultural considerations. Philos Ethics Humanit Med. 2014;9:19.
Erickson JA, Kuzmich L, Ormond KE, Gordon E, Christman MF, Cho MK, et al. Genetic testing of children for predisposition to mood disorders: anticipating the clinical issues. J Genet Couns. 2014;23:566–77.
Broome M, Fusar-Poli P. Philosophical issues in the prodromal phase of psychosis. Curr Pharm Des. 2012;18:596–605.
Ahmed E, Hens K. Microbiome in precision psychiatry: an overview of the ethical challenges regarding microbiome big data and microbiome-based interventions. AJOB Neurosci. 2022;13:270–86.
Gillett G, Saunders KEA. Remote monitoring for understanding mechanisms and prediction in psychiatry. Curr Behav Neurosci Rep. 2019;6:51–6.
Docherty A, Kious B, Brown T, Francis L, Stark L, Keeshin B, et al. Ethical concerns relating to genetic risk scores for suicide. Am J Med Genet PART B-Neuropsychiatr Genet. 2021;186:433–44.
Andlauer TFM, Nothen MM. Polygenic scores for psychiatric disease: from research tool to clinical application. Med Genet. 2020;32:39–45.
Parens E, Matthews L, Appelbaum PS. Polygenic risk scores, prediction of psychiatric disorders, and the health of all of us. Lancet Psychiatry. 2020;7:481.
Fusar-Poli P, Correll CU, Arango C, Berk M, Patel V, Ioannidis JPA. Preventive psychiatry: a blueprint for improving the mental health of young people. World Psychiatry. 2021;20:200–21.
Bloss CS, Jeste DV, Schork NJ. Genomics for disease treatment and prevention. Psychiatr Clin North Am. 2011;34:147–66.
Inglis A, Koehn D, McGillivray B, Stewart SE, Austin J. Evaluating a unique, specialist psychiatric genetic counseling clinic: uptake and impact. Clin Genet. 2015;87:218–24.
Costain G, Esplen MJ, Toner B, Hodgkinson KA, Bassett AS. Evaluating genetic counseling for family members of individuals with schizophrenia in the molecular age. Schizophr Bull. 2014;40:88–99.
Mundy J, Davies HL, Radu M, Austin J, Vassos E, Eley TC, et al. Research priorities in psychiatric genetic counselling: how to talk to children and adolescents about genetics and psychiatric disorders. Eur J Hum Genet. 2022. https://doiorg.publicaciones.saludcastillayleon.es/10.1038/s41431-022-01253-0.
Smith LB, Sapers B, Reus VI, Freimer NB. Attitudes towards bipolar disorder and predictive genetic testing among patients and providers. J Med Genet. 1996;33:544–9.
Gershon ES, Alliey-Rodriguez N. New ethical issues for genetic counseling in common mental disorders. Am J Psychiatry. 2013;170:968–76.
Turney J, Turner J. Predictive medicine, genetics and schizophrenia. New Genet Soc. 2000;19:5–22.
Pavarini G, Yosifova A, Wang K, Wilcox B, Tomat N, Lorimer J, et al. Data sharing in the age of predictive psychiatry: an adolescent perspective. Evid Based Ment Health. 2022;25:69–76.
Appelbaum PS. Ethical issues in psychiatric genetics. J Psychiatr Pract. 2004;10:343–51.
Potokar DN, Stein CH, Darrah OA, Taylor BC, Sponheim SR. Knowledge and attitudes about personalized mental health genomics: narratives from individuals coping with serious mental illness. Community Ment Health J. 2012;48:584–91.
Biesecker BB, Peay HL. Ethical issues in psychiatric genetics research: points to consider. Psychopharmacology. 2003;171:27–35.
Biesecker BB, Peay HL. Genomic sequencing for psychiatric disorders: promise and challenge. Int J Neuropsychopharmacol. 2013;16:1667–72.
Lerman C, Croyle RT, Tercyak KP, Hamann H. Genetic testing: psychological aspects and implications. J Consult Clin Psychol. 2002;70:784–97.
Sweeny K, Ghane A, Legg AM, Huynh HP, Andrews SE. Predictors of genetic testing decisions: a systematic review and critique of the literature. J Genet Couns. 2014;23:263–88.
Kvaale EP, Gottdiener WH, Haslam N. Biogenetic explanations and stigma: a meta-analytic review of associations among laypeople. Soc Sci Med. 2013;96:95–103.
Kvaale EP, Haslam N, Gottdiener WH. The ‘side effects’ of medicalization: a meta-analytic review of how biogenetic explanations affect stigma. Clin Psychol Rev. 2013;33:782–94.
Haslam N, Kvaale EP. Biogenetic explanations of mental disorder: the mixed-blessings model. Curr Dir Psychol Sci. 2015;24:399–404.
Colizzi M, Ruggeri M, Lasalvia A. Should we be concerned about stigma and discrimination in people at risk for psychosis? A systematic review. Psychol Med. 2020;50:705–26.
Buchman DZ, Imahori D, Lo C, Hui K, Walker C, Shaw J, et al. The influence of using novel predictive technologies on judgments of stigma, empathy, and compassion among healthcare professionals. AJOB Neurosci. 2024;15:32–45.
Hacking I. The looping effects of human kinds. In: Sperber D, Premack D, Premack AJ, editors. Causal cognition: a multidisciplinary debate. Oxford: Oxford University Press; 1996. p. 351–94.
Tsou JY. Hacking on the looping effects of psychiatric classifications: what is an interactive and indifferent kind? Int Stud Philos Sci. 2007;21:329–44.
Floridi L, Cowls J, Beltrametti M, Chatila R, Chazerand P, Dignum V, et al. AI4People—an ethical framework for a good ai society: opportunities, risks, principles, and recommendations. Minds Mach. 2018;28:689–707.
Beauchamp TL, Childress JF. Principles of Biomedical Ethics. 8th ed. Oxford. New York: Oxford University Press; 2019.
Ursin F, Timmermann C, Steger F. Explicability of artificial intelligence in radiology: is a fifth bioethical principle conceptually necessary? Bioethics. 2022;36:143–53.
Segers S, De Proost M. Take five? A coherentist argument why medical AI does not require a new ethical principle. Theor Med Bioeth. 2024;45:387–400.
Wang F, Kaushal R, Khullar D. Should health care demand interpretable artificial intelligence or accept “Black Box” Medicine? Ann Intern Med. 2020;172:59–60.
Grote T, Berens P. On the ethics of algorithmic decision-making in healthcare. J Med Ethics. 2020;46:205–11.
McDougall RJ. Computer knows best? The need for value-flexibility in medical AI. J Med Ethics. 2019;45:156–60.
Friedrich AB, Mason J, Malone JR. Rethinking explainability: toward a postphenomenology of black-box artificial intelligence in medicine. Ethics Inf Technol. 2022;24:8.
Word Health Organization. WHO report highlights global shortfall in investment in mental health. https://www.who.int/news/item/08-10-2021-who-report-highlights-global-shortfall-in-investment-in-mental-health. Accessed 21 Aug 2024.
Acknowledgements
We would like to thank Esther Mesman for providing valuable input into the methodology of this study
A previous version of this study was presented at the 35-th European Conference on Philosophy of Medicine and Health Care in Riga, 2023. We would like to thank the audience for suggestions how to improve the study.
Funding
This research has received funding from the FAMILY project under the European Union’s Horizon Europe research and innovation programme (grant number 101057529), the Swiss State Secretariat for Education, Research and Innovation (SERI) and the UK Research and Innovation (UKRI) under the UK government’s Horizon Europe funding guarantee'. Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union, or the European Health and Digital Executive Agency (HADEA), the SERI or the UK Research and Innovation (UKRI). Neither the European Union nor the granting authorities can be held responsible for them.
Author information
Authors and Affiliations
Contributions
IN, SM and NH contributed to the study design. IN and SM performed data retrieval and coding for analysis. All authors contributed to the analysis and interpretation of the articles included in the review. IN and SM wrote the first draft of the paper. All authors reviewed the manuscript critically. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Neiders, I., Mežinska, S. & van Haren, N.E.M. Ethical and social issues in prediction of risk of severe mental illness: a scoping review and thematic analysis. BMC Psychiatry 25, 501 (2025). https://doiorg.publicaciones.saludcastillayleon.es/10.1186/s12888-025-06949-3
Received:
Accepted:
Published:
DOI: https://doiorg.publicaciones.saludcastillayleon.es/10.1186/s12888-025-06949-3